- Conditions
- Procedures
- Patient care
- Why choose us
- Our Doctors
- Contact
Home » Spine Procedures »
Spinal Cord Stimulator
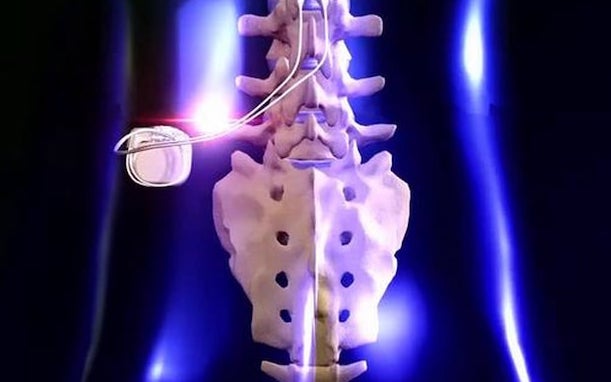
What is a spinal cord stimulator?
A spinal cord stimulator is a small device that is surgically implanted directly into the spine that helps to relieve pain and other symptoms through gentle electrical impulses. Since the spinal cord is responsible for transmitting sensory and motor information from the brain to the rest of the body, injuries and complications can result in debilitating pain and severe mobility problems.
Spinal cord stimulators have been developed as a treatment for patients who have exhausted conservative treatment options without finding relief from spinal cord pain. The team at USA Spine Care wants to help patients learn about the wide array of treatment options that are available that can lead to improved quality of life and lasting pain relief. If you have questions or want more information as you read over the following information, please contact us at any time.
What conditions are spinal cord stimulators used for?
The spine is intricately constructed to protect the spinal cord, but it is also tasked with supporting the upper and lower body and allowing for movement and flexibility. This puts the spinal cord and surrounding nerves at risk for compression and other injuries. Although not every spine condition is best treated with a spinal cord stimulator, commonly treated conditions include:
- Chronic back pain and leg pain due to age-related conditions like arthritis or degenerative disc disease
- Complications related to previous spine surgery, also known as failed back surgery syndrome (FBSS)
- Arachnoiditis, or painful inflammation of the thin membrane that covers the brain and spinal cord
- Neuropathy related to diabetes
- Complex regional pain syndrome, a progressive condition that causes pain in the extremities
While medical researchers are still working to fully understand the mechanisms of spinal cord stimulation, there is strong evidence that this is an effective form of treatment for many patients dealing with chronic pain.
Spinal cord stimulator procedure and recovery overview
Before receiving a permanent spinal cord stimulator, patients will typically first undergo a trial procedure to determine if it will be effective. A spinal cord stimulator consists of two electrodes and a battery pack similar to a pacemaker. For the trial procedure, a single tiny incision is made to insert the electrodes into the epidural space of the spine while the battery remains outside of the body. Over a short period of time, patients can determine if spinal cord stimulation helps them experience long-term relief.
For the permanent implantation, the electrodes will be replaced with new ones that the surgeon anchors to the body and the battery pack will be implanted safely under the skin, often near the base of the spine. This is still performed as an outpatient procedure and generally only takes one to hours. Recovery is minimal, with the incision healing in about two to four weeks, with doctors recommending light activity for the first couple of weeks.
Spinal cord stimulator cost 2020-2021
The cost for a spinal cord stimulator procedure depends on a wide array of factors, including the device being used and the insurance carrier. Medicare patients should be aware that spinal cord stimulators have been approved under CPT code 63650. USA Spine Care works with Medicare and most private health insurances, as well as workers’ compensation and personal injury cases. Our team can answer any questions about cost.
Reach out to the experts at USA Spine Care today
If you’ve exhausted conservative treatment options and chronic pain is reducing your ability to spend time with your family and perform at your job, spinal cord stimulation could be a potential treatment option. Our world class surgical team at USA Spine Care has decades of experience helping patients find the relief they deserve through spinal cord stimulation and other treatment options.
To learn more, contact our dedicated team today.
Call toll free 1- 866-249-1627.
Spinal Cord Stimulator - People Also Ask
Spinal cord stimulator implants are covered by Medicare and are billed under:
- Percutaneous Leads and Extensions
63650 Percutaneous implantation of neurostimulator electrode array, epidural
63661 Removal of spinal neurostimulator electrode percutaneous array(s), including fluoroscopy, when performed
63663 Revision including replacement, when performed, of spinal neurostimulator electrode percutaneous array(s), including fluoroscopy, when performed - Paddle Leads
63655 Laminectomy for implantation of neurostimulator electrodes, plate/paddle, epidural
63662 Removal of spinal neurostimulator electrode plate/paddle(s) placed via laminotomy or laminectomy, including fluoroscopy, when performed
63664 Revision including replacement, when performed, of spinal neurostimulator electrode plate/paddle(s) placed via laminotomy or laminectomy, including fluoroscopy, when performed - Stimulators
63685 Insertion or replacement of spinal neurostimulator pulse generator or receiver, direct or inductive coupling
63688 Revision or removal of implanted spinal neurostimulator pulse generator or receiver - Analysis and Programming
CPT codes 95970–95973 are used to report electronic analysis services. These are not considered medically necessary when provided at a frequency more often than once every 30 days. More frequent analysis may be necessary in the first month after implantation.
Published studies of spinal cord stimulation show good to excellent long-term relief in 50 to 80% of patients suffering from chronic pain. One study reports that 24% of patients improved sufficiently to return to gainful employment or housework with stimulation alone or with the addition of occasional oral pain medication.
Unlike a spinal fusion, a spinal cord stimulator surgery is reversible. If a patient decides at any time to discontinue, the electrode wires and generator can all be removed.
Spinal Cord Stimulation is an option for those suffering from chronic, intractable pain of lower back and/or limbs including unilateral or bilateral pain associated with the following conditions:
- Failed Back Surgery Syndrome (FBSS)
- Radicular pain
- Post-laminectomy pain
- Multiple back surgeries resulting in continued or worsening pain
- Unsuccessful vertebral disk surgery
- Degenerative Disk Disease
- Peripheral causalgia
- Epidural fibrosis
- Arachnoiditis
- Complex Regional Pain Syndrome (CRPS)
- Causalgia
No surgery is without risks. General complications of any surgery include bleeding, infection, blood clots, and reactions to anesthesia. Specific complications related to SCS may include:
- Undesirable changes in stimulation (can possibly be related to cellular changes in tissue around electrodes, changes in electrode position, loose electrical connections, and/or lead failure)
- Epidural hemorrhage, hematoma, infection, spinal cord compression, and/or paralysis (can be caused by placing a lead in the epidural space during a surgical procedure)
- Battery failure and/or battery leakage
- Cerebrospinal fluid leak
- Persistent pain at the electrode or stimulator site
- A pocket of clear fluid (seroma) at the implant site. Seromas usually disappear by themselves but may require a drain.
- Lead migration, which can result in changes in stimulation and reduction in pain relief
- Allergic response to implant materials
- Generator migration and/or local skin erosion
- Paralysis, weakness, clumsiness, numbness, or pain below the level of implantation
In a spinal cord stimulator trial, temporary electrodes are placed and then the patient uses an external device to generate electrical current. The electrodes are placed under x-ray guidance with the patient lying on his belly. A local anesthetic is used to numb the skin and deeper tissues. The electrical wire or lead contains a series of four to eight evenly spaced electrodes that can be programmed to generate an electrical field. A spinal cord stimulator trial period is at least 5 to 7 days. This gives you time to test the device and evaluate its effectiveness managing your pain at rest and during activity
